組織採樣是檢測準確度的關鍵,盡可能採取最大的組織樣本1,2
|
Limited tissue availability1
|
Sampling site: metastases are preferred2
|
- Single sampling generates a relatively small amount of tissue
- Sample may contain limited tumor tissue because of the heterogeneity of NSCLC tissue
- Only one opportunity to fix and process the tissue
- Amount of tissue available for molecular testing may be limited by priorities of testing:
- Histologic diagnosis
- IHC for tumor classification
- Molecular testing
|
- Sampling metastases ensures that the more important fraction of total tumor cell burden is collected
- This approach also avoids potential heterogeneity problems within the primary tumor
|
建議事項:
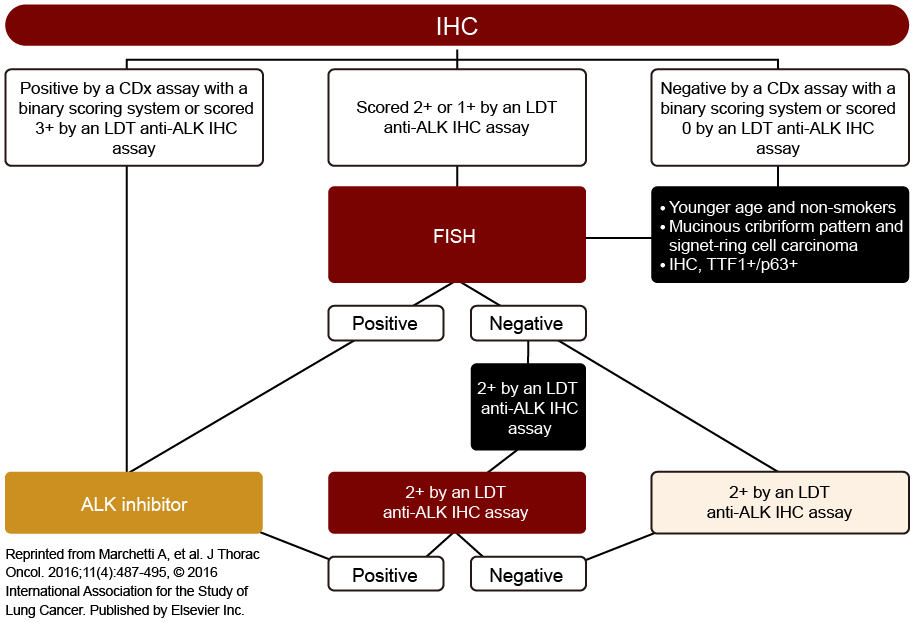
- A number of proposed algorithms have been published based on the advantages and characteristics of individual testing methods1-3
- There is no standard diagnostic algorithm1
- Shown to the right is one example of a proposed algorithm designed to prevent misdiagnoses3
FDA核准的檢測方法:
|
Vysis ALK Break Apart FISH Probe
|
- Vysis ALK Break Apart FISH Probe Kit
- First FDA-approved companion diagnostic test for detection of ALK rearrangements in NSCLC
- Detects ALK rearrangements regardless of fusion partner
|
|
Ventana ALK (D5F3) IHC CDx Assay Staining
|
- Ventana ALK (D5F3) CDx Assay
- Fully automated IHC diagnostic test
- FDA-approved diagnostic test for ALK rearrangements (June 2015); previously approved in Europe (2012) and China (2013)
|
|
FoundationOne CDxTM
|
- Approved comprehensive genomic profiling test for solid tumor cancers
- NGS-based in vitro diagnostic that incorporates multiple companion diagnostics including
detection of ALK rearrangements in NSCLC
|
|
MSK-IMPACTM
|
- First FDA-approved laboratory-developed test (2017)
- NGS-based in vitro diagnostic with a 468-gene oncopanel, including ALK rearrangements
|
|
Oncomine Dx6
|
- FDA-approved targeted NGS test for NSCLC that detects 368 variants in 23 cancer-associated
genes including ALK mutations, but not ALK fusions/translocations
|
FDA=US Food and Drug Administration; MSK-IMPACT=Memorial Sloan Kettering Integrated Mutation Profiling of Actionable Cancer Targets; ALK=anaplastic lymphoma receptor tyrosine kinase; NSCLC=non–small cell lung cancer; IHC= immunohistochemical; CDx assay=companion diagnostic assay; FISH=fluorescence in situ hybridization; LDT=laboratory-developed test; NGS=next-generation sequencing; RT-PCR=real-time polymerase chain reaction
