ALK 基因的檢測方法
判斷 NSCLC 病人是否屬於 ALK+ ,包含以下幾種檢測方法:FISH, IHC, NGS, RT-PCR, Liquid Biopsy1
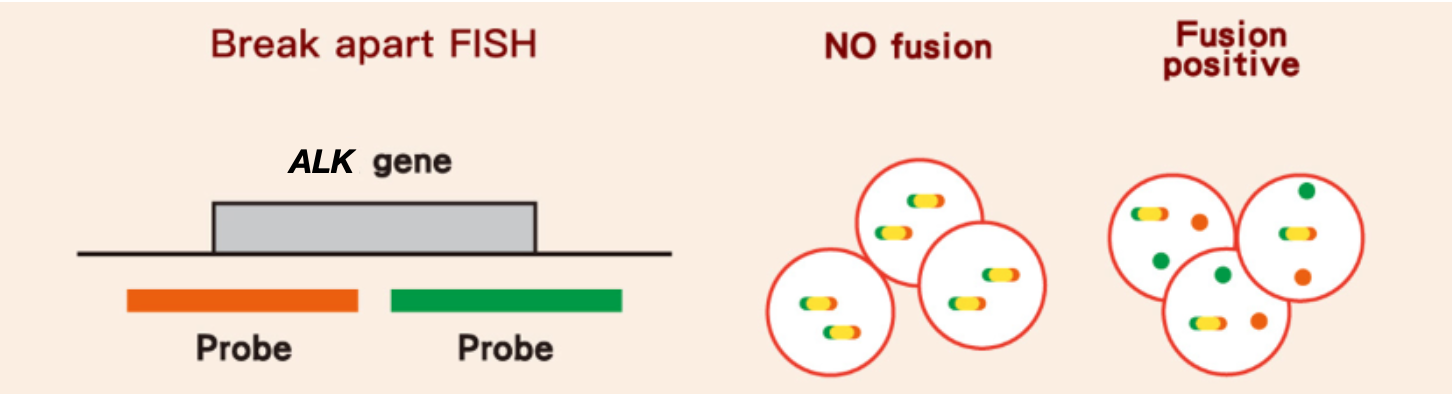
Fluorescence in situ hybridization (FISH) 2
| Utilization |
Limitations |
- FISH is currently a standard method for ALK testing
- The use of FISH with a break-apart probe can detect ALK translocations, regardless of the variant
|
- Potential for false-negative and false-positive results is a primary challenge
- Depends heavily on careful tissue preparation
- Must be interpreted with strict adherence to guidelines
- Expensive
|
螢光原位雜合技術 (Fluorescence in situ hybridization, FISH) 是 FDA 核准作為 ALK
檢測的方法之一3,受限於樣本處理的狀況,可能出現偽陽性或偽陰性反應,且費用高,檢測耗時,並非每一間檢驗中心都具備相關技術和儀器可以進行;另一個類似的檢測方式顯色原位雜合技術
(Chromogenic in situ hybridization,
CISH)4,原理類似,只是呈色方式不同,兩者皆有相同的限制,即無法區分 ALK
亞型,且無法觀察腫瘤組織的型態結構。
Representative FISH images of ALK-negative and positive tumors5
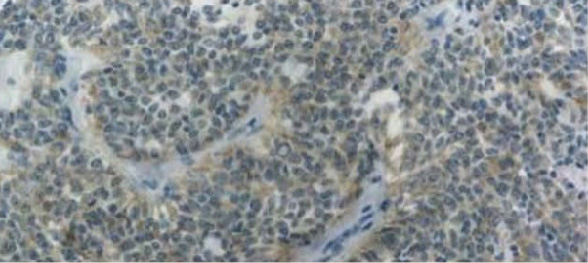
Immunohistochemistry (IHC)2
| Utilization |
Limitations |
- Antibodies against ALK protein can detect overexpression
- IHC is the most popular screening method due to many factors:
- Rapid
- Cost-effective
- Requires fewer malignant cells than needed for FISH
- Is effective on a variety of different tumor specimen types, including routinely prepared formalin-fixed, paraffin-embedded (FFPE) specimens
|
- Dependent on the quality of the antibody and the level of ALK protein
- Confirmation of positive results on IHC by FISH is encouraged, although FDA-approved IHC (ALK [D5F3] CDx Assay) can be used as a stand-alone test
- Potential for false-negative and false-positive results
|
免疫組織染色 (Immunohistochemistry, IHC) 是以 ALK 抗體辨識組織切片上的 ALK 蛋白,再進行呈色反應確認 ALK 蛋白是否過量表現。免疫組織染色是最受歡迎的檢測法,費用較低且更快得到結果,只需要少量檢體且需要使用的設備也較簡單,石蠟包埋的組織 (formalin-fixed paraffin-embedded, FFPE) 也可以進行該檢測。該檢測結果同樣會有偽陰性和偽陽性反應,且篩檢方式尚未標準化,抗體的品質和檢體內 ALK 的表現量也會影響檢測結果,因此檢測結果為陽性的組織建議再以螢光原位雜合技術雙重確認檢測結果。
Positive ALK-IHC5
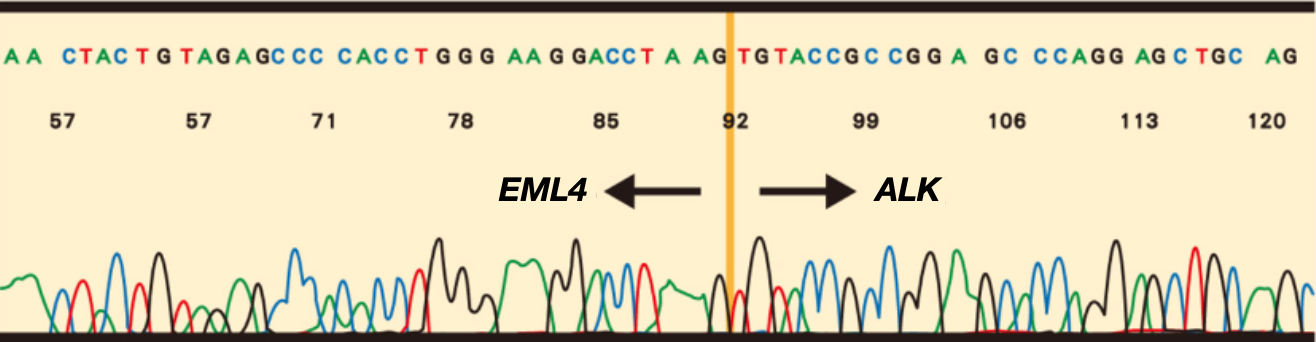
Reverse transcriptase – polymerase chain reaction (RT-PCR) 1,2,6
| Utilization |
Limitations |
- The use of multiplex PCR primers allows detection and identification of specific translocations
- Provides detailed genetic information; is highly reliable (high specificity, no false-positives)
- Very low copy numbers of RNA can be detected
|
- Requires isolating high-quality RNA, which is difficult to obtain in clinical practice
- Not recommended by guidelines: risk of false-negative results and high failure rate for RNA-based assays on FFPE samples
- May be adequate for confirming results of IHC and FISH analyses; less appropriate for primary screening for ALK rearrangement—especially when the amount of tissue sample is adequate
|
反轉錄 PCR (RT-PCR) 只能檢測已知的基因序列,專一性高且不容易出現假陽性反應,只需要非常少量就可以檢測出來,缺點是對於不常見的基因重組序列,可能因此沒有被檢測出來,且 RNA 容易降解,檢體保存的方式或萃取的過程都可能影響其質量,造成檢測準確度的變異。該檢測法需要分離出高質量的 RNA,假陰性的機率高,因此不被指引建議為主要檢測方式,比較常用於雙重確認 FISH 或者 IHC 的檢測結果。
The chimeric transcript was detected with RT-PCR products5
Next-generation sequencing (NGS)7-8
| Utilization |
Limitations |
- Multiplex testing of large number of genes with one single test
- Detects different types of mutations, including deletions, insertions, copy number alterations, and rearrangements
- NGS has been shown to identify ALK-rearranged NSCLC patients not detected by FISH
- If IHC is inconclusive, sequencing with NGS can be considered
|
- NGS requires more tissue, time for analysis, and is more expensive than FISH and IHC
- New technology with few studies evaluating NGS concordance with established diagnostic assays
|
次世代基因定序 (Next-generation sequencing, NGS) 可以發現 FISH 未檢測的 ALK+ NSCLC,但是需要的檢體量更大,分析時間更長,費用也更高。Oncomine Dx Target Test作為第一個FDA核准的NGS,所檢測的就是非小細胞肺癌,其中包含 EGFR, ALK, ROS1, MET 幾項已知驅動變異基因且已經發展出相對應的標靶藥物。
Liquid Biopsy
- Provides genetic information on primary tumors and metastases9
- Noninvasive and easy to repeat9
- Rapid turnaround time9
- Higher chance of false-negatives compared with traditional biopsies, due to the small and variable amounts of DNA that may be shed into circulation11
- Large gene rearrangements such as translocations are challenging to detect by liquid biopsy9
- Currently not FDA-approved for ALK testing11
|
NSCLC=non–small-cell lung cancer; ALK=anaplastic lymphoma receptor tyrosine kinase; FDA=US Food and Drug Administration; FISH=fluorescence in situ hybridization; CISH=chromogenic in situ hybridization; IHC=immunohistochemistry; FFPE=formalin-fixed paraffin-embedded; RT-PCR=reverse transcriptase – polymerase chain reaction; PCR=polymerase chain reaction; NGS=next-generation sequencing
